Carbon, often dubbed the ‘element of life,’ holds a unique place in the universe. From the dazzling allure of diamonds to the very blueprint of life, DNA, carbon’s reach is vast and varied. It’s both the ink in our pens and the fuel for our fires. Yet, many of its tales remain untold!
Carbon has three forms: amorphous, graphite, and diamond. The element carbon in the form of carbon dioxide is our planet’s most important greenhouse gas because it absorbs and radiates heat from the Earth’s surface and then sends it back toward Earth’s surface and to other places, which can cause global warming if we produce too much carbon dioxide.
It’s best known as a gas that needs to be absorbed by plants and trees so it doesn’t float into the atmosphere and contribute to the greenhouse effect by warming the planet. Burning gasoline produces carbon monoxide. We need to decrease fossil fuels and increase the use of sustainable fuels to reduce global warming and protect trees.
Understanding Carbon

Most life forms need carbon dioxide to maintain a habitable temperature, but adding an excess of carbon dioxide accelerates the natural greenhouse effect. In 2021, carbon dioxide was solely responsible for about two-thirds of the total heating influence of human-produced greenhouse gases.
The carbon cycle is the process in which atoms travel from the atmosphere to our planet and then back into the atmosphere and involves the conversion of atmospheric carbon dioxide to carbohydrates by photosynthesis in plants.
Carbon is in many items, has a variety of uses, provides a major source of energy to fuel the global economy, and is the fourth most abundant of the elements.
What is Carbon?
The carbon chemical element is a lot more interesting than we ever imagined! It’s the glue of life on Earth, and it makes up around a fifth of our bodies (almost 19% carbon by weight). It’s in organic material like DNA, proteins, and other human body parts.
Organic material is made from organic matter that comes from the remains of organisms such as plants and animals and their waste products in the environment.
More interesting facts: When living organisms are formed, carbon makes protein and DNA. It’s the fourth most abundant element in the universe and the 15th most abundant on Earth.
Carbon forms strong bonds. It’s the foundation for most life on Earth, and scientists use it to calculate the age of organic materials.
Plants need carbon monoxide for photosynthesis, but carbon monoxide, a one-carbon atom with one oxygen atom, is a colorless and odorless gas and toxic to both humans and animals. Carbon monoxide also is used in metal fabrication.
Carbon Facts
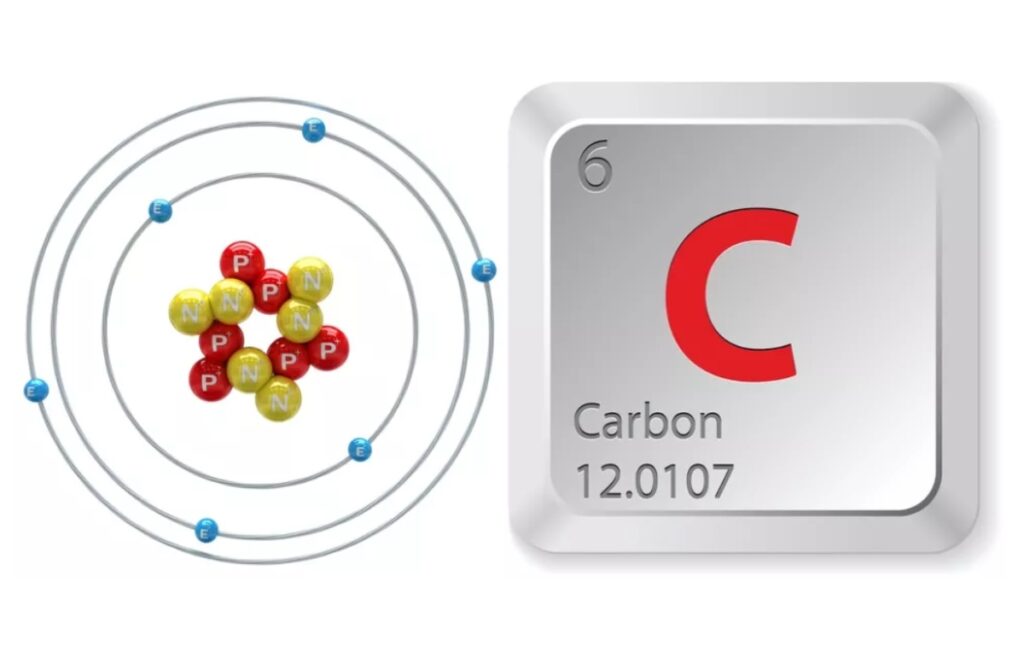
Atomic Number of Carbon is 6
Carbon’s number is six because each atom has six protons, six neutrons, and six electrons–placing it at the sixth position on the periodic table.
Atomic Symbol of Carbon is C
Carbon was named from the Latin word “carbo,” meaning coal, which is made of carbon and other elements. Its periodic table symbol is C.
Atomic Weight of Carbon is 12
The mass of carbon atoms on the periodic table is 12.01 amu, meaning that it’s the average atomic mass.
The Density of Carbon is 2.2670 Grams per Cubic Centimeter
The density of carbon is 2.2 g.cm-3 at 20°C.
Total Number of Isotopes is 15
There are 15 carbon isotopes, three are naturally occurring, and the rest are manmade. Carbon-12 and carbon-13 are stable isotopes, but carbon-14 is unstable and radioactive.
Most Common Isotopes of Carbon Are 3
Carbon occurs naturally in C12, C13, and C14. The first two are two stable isotopes. Isotopes are atoms of the same element with a different number of neutrons.
Carbon Has a Valency of +4
Valency is the combining power of an element with hydrogen, and it’s calculated by the number of hydrogen bonds that attach to an element to give the valency. The outer shell of carbon is four, so carbon makes four bonds with hydrogen.
Melting Point: 6,422 Degrees Fahrenheit (3,550 Degrees Celsius)
Each carbon atom is covalently bonded to four other carbon atoms, so a great deal of energy is needed to separate the carbon atoms in a diamond. Covalent bonds are strong, and diamonds contain many covalent bonds.
Boiling Point: 6,872 Degrees Fahrenheit (3,800 Degrees Celsius) – Sublimation
Carbon’s boiling point is extremely high because of its very strong non-covalent interactions between molecules, requiring more heat energy to break them apart. Higher melting and boiling points mean stronger non-covalent intermolecular forces.
Fun Facts About Carbon
Despite being made of carbon, many of us aren’t that familiar with it. Here are some facts about elemental carbon:
The Name “Carbon” Is Derived from the Word Coal
Due to the high content of carbon in coal, scientists named the word carbon after the Latin word for coal, “carbo.”
Carbon Ranks among the Most Prevalent Elements in the Cosmos
Hydrogen is the most prevalent element, helium ranks number two, oxygen is third, and carbon is fourth.
Carbon Is Sixth on the Periodic Table and is Between Boron and Nitrogen
It’s sixth due to having six protons, six neutrons, and six electrons. Nitrogen is the most common gas in our atmosphere. Nitrogen is extremely important to living material.
Carbon Comprises a Weight of .032% in the Earth’s Lithosphere
The lithosphere is the rigid outer part of the earth, made of the earth’s crust and upper mantle. Carbon is stored in the lithosphere and weighs 0.032% of the lithosphere in our Earth’s crust.
The Fourth Most Abundant Element in the Universe is Carbon
Carbon is the fourth most abundant element in the universe, with 0.05%. The universe is 73.92% Hydrogen, 24.08% Helium, and 1.06% Oxygen.
Almost .04% of the Earth’s Atmosphere is Made up of CO2
The earth’s atmosphere consists of 0.04% C02. In 2019, humans sent 36.44 billion tonnes of CO2 into the atmosphere. That CO2 traps heat in the atmosphere.
Carbon Exists Mainly as Carbon-12, Comprising Nearly 99% of the Universe’s Carbon
Carbon 12 is almost 99% of the universe’s carbon. Carbon 12 is used as a standard for measurements of atomic weight.
Carbon Readily Forms Bonds with Other Elements
Elemental carbon is able to form bonds with other elements very easily because it has four electrons in its outer shell that enable it to attach to other atoms. It’s a pattern maker that can link to itself while forming long, resilient chains of polymers (a molecule of repeated units).
Other Forms of Carbon
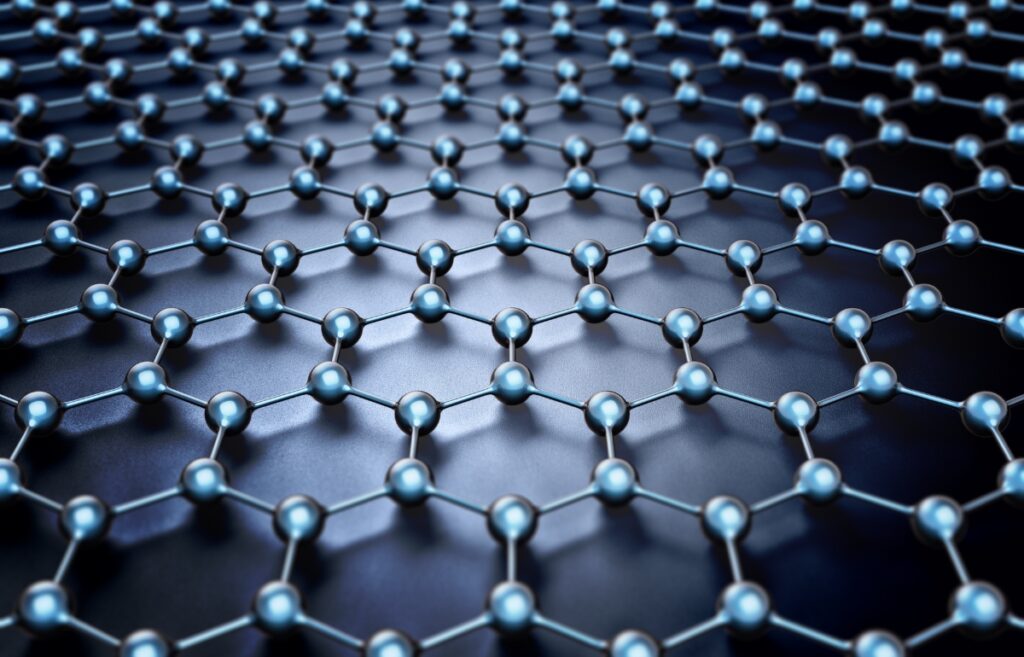
Other types of carbon are graphene–the strongest material, fullerene, glassy carbon, carbon nanofoam, and Q-carbon (which is magnetic and fluorescent). Graphene, a new form of carbon, was discovered accidentally in 2004 by Andre Geim and Kostya Novoselov, and it won them a Nobel Prize.
It’s extremely thin and resilient, stronger than steel, more flexible than rubber, and conducts electricity.
Carbon Is an Ingredient in Many Products and Substances
Hydrocarbons make natural gas, propane, diesel, kerosene, gasoline, jet fuel, and coal. Those all cause pollution. They are used to make synthetic fabrics, plastics, medications, and polyester. Plastics are also made from Carbon polymers. The simplest hydrocarbon compound is methane.
Australian doctor John Cade used a 0.5% solution of lithium carbonate to calm a mentally disturbed patient in 1949, and the lithium solution is still used.
The triple alpha process happens when stars fuse together three particles and make a new particle with six protons, six neutrons, and three helium nuclei fuse. Each alpha particle contains two protons and two neutrons. This is the world’s most common form of carbon.
Carbon compounds such as carbonates of magnesium and calcium make common minerals like magnesite, dolomite, marble, and limestone. Calcium makes coral and the shells of oysters and clams carbonate.
Roughly One-fifth of the Human Body Consists of Carbon (News Medical)
More than 90% of this tissue CO2 is stored in bone as carbonate, and it’s also in other areas of the human body.
Carbon Is the Main Ingredient of Organic Matter, Including Dna and Proteins
When combined with other substances, carbon is in a lot of important matter, including sugars, starches, fats, oils, enzymes, antibodies, DNA, and RNA. Carbon is a key ingredient in the foods we eat.
Diamonds and Graphite: Hardest and Softest Natural Materials
The Mohs Hardness Scale measures the hardness of minerals to each other. One is the softest, and ten is the hardest. Graphite is soft and comes in at one or two on Mohs. Diamonds, which are formed under high pressure and are the hardest natural substance, score 10.
The difference between the two is their crystal structure. A diamond has four atoms joined to four other atoms. They have different properties because of the way the atoms in their structures are arranged.
Diamond films resist wear 10 times longer and can be used for protective coatings, ball bearings, metal cutting tools, and optical instruments and systems, as well as consumer products.
Graphite is used to make lubricants, polishes, batteries, crucibles, foundry facings, polishes, arc lamps, and brushes for electric motors. Graphite is also used for the cores of nuclear reactors.
Diamonds Are Dubbed ‘ice’ for Their Heat-conducting Cool Touch and Not Appearance
People sometimes think that a diamond is referred to as ice because of its clarity resembling actual ice. However, ice refers to their heat-conducting cool touch. A diamond is perfect for applications conducting heat and sustaining intensely high temperatures and electric fields. The diamond has a number of potential applications due to its strength.
In 1772, Antoine Lavoisier used a solar furnace to burn a diamond in a glass jar, and he was able to show that the diamond consisted exclusively of carbon. Imagine everyone’s surprise that a beautiful diamond was made of carbon!
Graphene Stands as the Thinnest and Strongest Substance Ever Discovered
Graphene is one layer of carbon fashioned in a hexagon lattice of 2D electron confinement in a one-atom-thick layer. It’s used for next-generation technology for wearables and really fast electronics, composites and coatings, sensitive sensors, membranes, biology, energy harvesting, medicine, and storage.
While Pure Carbon Is Generally Deemed Safe, Inhaling Fine Particles like Soot Can Harm Lung Tissues
Environmental carbon is safe to breathe, but carbon blacks (CB) and environmental soot are formed by the incomplete combustion of hydrocarbons, which are organic compounds but have different percent carbon contents. They cause diseases in humans. Organic chemistry is the study of hydrocarbons.
In 1775, a London surgeon called Sir Percival Pott, observed that many chimney sweeps developed scrotal cancer and connected soot with toxins. Amorphous (a free reactive carbon without crystal structure) carbons like coal, soot, etc.; diamond, and graphite are the three best-known types of carbon and are called allotropes.
Graphite and clay are used to make lead for pencils, and graphite is a good conductor of electricity.
The First Compound Used for Tattooing Was Carbon Black
CBs are also found as a pigment intattoos, which may be carcinogenic. That’s one of the unpleasant facts about carbon.
Coal Uses
Coal is used for making steel for bridges and buildings and for heat and electricity.
Charcoal Uses
Charcoal has three main uses: to make gunpowder, to decolorize sugar, and to make gas masks because it absorbs harmful gases. Also, the beauty industry has been touting activated charcoal.
According to a Study, Buckyballs Have the Ability to Inhibit Hiv
Another interesting fact about carbon is Fullerene, a molecule of carbon in the shape of a hollow sphere, tube, ellipsoid, and many other forms. Spherical fullerene, also known as Buckminsterfullerenes or buckyballs, resemble soccer balls. Their structure is similar to graphite, which is stacked graphene sheets of linked hexagonal shapes.
Russian researchers found a potent anti-HIV activity of the alkoxy fullerene derivative that may possibly be taken orally because it’s water‐soluble but is still being tested.
Carbon Is Key for Next-generation Tech-related Materials
More interesting facts include that carbon nanotubes play a big role in the field of electronics. It’s expensive right now, but its ability to conduct electricity and heat, its strength, and its light weight make it perfect for transistors, heat dissipation in electronic devices, and for supercapacitors.
Carbon nanotubes can be used to create stronger, more durable, and lighter devices, including wearable tech, laptops, and smartphones. Nanotechnology is the future, and nanotubes have incredible capabilities.
Hydrocarbons Are Organic Carbon Compounds
Hydrocarbons are made entirely of just hydrogen and carbon.
It Was Involved in the Big Bang
Hydrogen and helium are theorized to have been formed during the ‘Big Bang,’ and carbon is believed to have come from an accumulation of alpha particles in supernova explosions. Talk about interesting facts!
The Carbon 14 isotope is radioactive and has a half-life of about 5,730 years, meaning that half the atoms in a sample will change into other atoms or decay in that time. Carbon 14 is used for dating organic materials back to around 60,000 years ago.
Carbon 14 was discovered in the 1940s, and scientists have been using Carbon 14 to date materials since then. Usually, a half-life is very long. Plants take carbon 14 up in respiration.
Rocks and Minerals

Carbon combines with oxygen and other substances to make non-living items like rocks and minerals. Who knew there’s carbon in rocks and minerals?
Places where Carbon is stored
Carbon is stored in seawater and coastal ecosystems because they absorb and store carbon at a much faster rate than other areas, such as forests–another reason to protect the oceans.
Amorphous carbon is free, reactive carbon that does not have any crystalline structure.
Soda and Paint Ball Guns
Two final interesting facts, carbon molecules are in soda (carbonated) and paintball guns.
Properties of Carbon
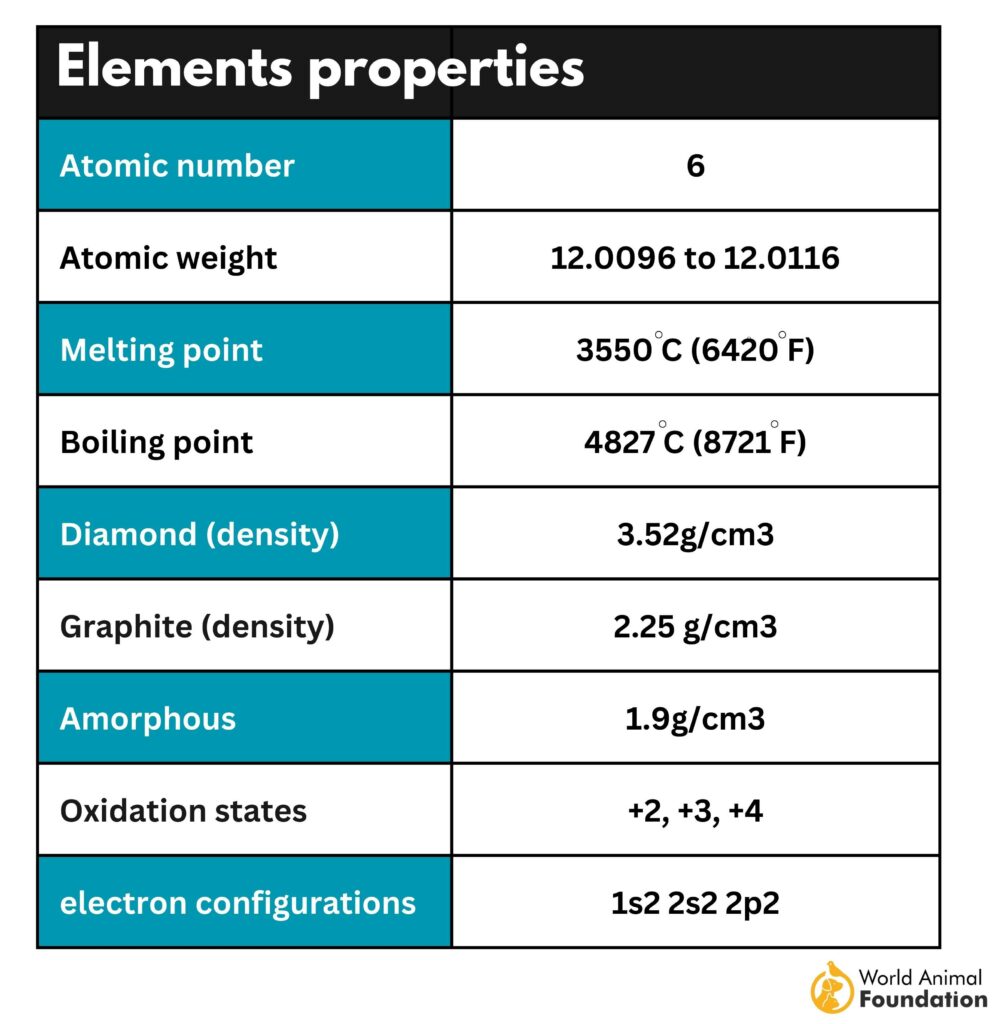
Carbon Physical Properties
It’s black or dull gray and delicate or soft. Carbon has several allotropes (different forms of pure chemical elements).
Chemical Properties of Carbon
- Atomic number: 6
- Atomic mass: 12.011 g.mol -1
- Electronegativity according to Pauling: 2.5
- Density: 2.2 g.cm-3 at 20°C
Carbon Periodic table
Atomic number 6, nonmetal, solid at room temperature.
Carbon Structure
Six protons, six neutrons, and six electrons
Carbon Uses
Carbon in the form of charcoal (from wood) and coke (from coal) is used in metal smelting for the iron and steel industries. Graphite is used in pencils.
FAQs
Is Carbon an Element or Compound?
Element
When Was Carbon Discovered?
1772
What Is the Carbon Atomic Number?
6
What Number Is Carbon on the Periodic Table?
6
What Is the Melting Point of Carbon?
6,422 degrees Fahrenheit
How Was Carbon Discovered?
Lavoisier burnt a diamond in a glass jar in a solar furnace, and he was able to demonstrate that diamond consisted exclusively of carbon because all that was left of the diamond was carbon.
Conclusion
Capping off our exploration into carbon, it’s evident that this elemental gem is far more than meets the eye. With a presence in our every breath to the depths of the cosmos, carbon is the thread weaving together life and wonders beyond imagination.
As we continue to unearth its stories, one fact stands clear: carbon’s tapestry in the universe is both intricate and infinitely vast.


